Downloads: 1
Switch to side-by-side view
--- a/README.md +++ b/README.md @@ -1,118 +1,118 @@ -# scDEC - -[](https://zenodo.org/badge/latestdoi/286327774) - - - -scDEC is a computational tool for single cell ATAC-seq data analysis with deep generative neural networks. scDEC enables simultaneously learning the deep embedding and clustering of the cells in an unsupervised manner. scDEC is also applicable to multi-modal single cell data. We tested it on the PBMC paired data (scRNA-seq and scATAC-seq) from 10x Genomics (see Tutorials). - -## Recent News - -An modified version of scDEC won the first place in [NeurIPS 2021 Multimodal Single-Cell Data Integration competition](https://openproblems.bio/neurips_2021/) two Joint Embedding tasks. - -## Requirements -- TensorFlow==1.13.1 -- Scikit-learn==0.19.0 -- Python==2.7 - -## Installation -Download scDEC by -```shell -git clone https://github.com/kimmo1019/scDEC -``` -Installation has been tested in a Linux platform with Python2.7. GPU is recommended for accelerating the training process. - -## Instructions - -This section provides instructions on how to run scDEC with scATAC-seq datasets. One can also refer to [Codeocean platform](https://codeocean.com/capsule/0746056) and click `Reproducible Run` on the right. The embedding and clustering results of several datasets will be shown on the right panel. - -### Data preparation - -Several scATAC-seq datasets have been prepared as the input of scDEC model. These datasets can be downloaded from the [zenode repository](https://zenodo.org/record/3984189#.XzDpJRNKhTY). Uncompress the `datasets.tar.gz` in `datasets` folder then each dataset will have its own subfolder. Each dataset will contain two major files, which denote raw read count matrix (`sc_mat.txt`) and cell label (`label.txt`), respectively. The first column of `sc_mat.txt` represents the peaks information. - -### Model training - -scDEC is an unsupervised learning model for analyzing scATAC-seq data. One can run - -```python -python main_clustering.py --data [dataset] --K [nb_of_clusters] --dx [x_dim] --dy [y_dim] --train [is_train] -[dataset] - the name of the dataset (e.g.,Splenocyte) -[nb_of_clusters] - the number of clusters (e.g., 6) -[x_dim] - the dimension of Gaussian distribution -[y_dim] - the dimension of PCA (defalt: 20) -[is_train] - indicate training from scratch or using pretrained model - -``` -For an example, one can run `CUDA_VISIBLE_DEVICES=0 python main_clustering.py --data Splenocyte --K 12 --dx 8 --dy 20` to cluster the scATAC-seq data with pretrained model. Note that the dimension of the embedding should be `K+x_dim` - -Or one can run `CUDA_VISIBLE_DEVICES=0 python main_clustering.py --data Splenocyte --K 12 --dx 8 --dy 20 --train True` to train the model from scratch. - -### Model evaluation - -If the pretrained model was used, the clustering results in the last step will be directly saved in `results/[dataset]/data_pre.npz` where `dataset` is the name of the scATAC-seq dataset. Note that `data_pre.npz` or `data_at_xxx.npz` contains the predictions from the H network. The first part denotes the embeddings and the second part denotes the inferred one-hot label where one can use `np.argmax` function to get the cluster label. - -Then one can run `python eval.py --data [dataset]` to analyze the clustering results. -For an example, one can run `python eval.py --data Splenocyte` - -The t-SNE visualization plot of latent features (`scDEC_embedding.png`), latent feature matrix (`scDEC_embedding.csv`), inferred cluster label (`scDEC_cluster.txt`) will be saved in the `results/[dataset]` folder. - - -If scDEC model was trained from scratch, the results will be marked by a unique timestamp YYYYMMDD_HHMMSS. This timestamp records the exact time when you run the script. The outputs from the training includes: - - 1) `log` files and predicted assignmemnts `data_at_xxx.npz` (xxx denotes different epoch) can be found at folder `results/[dataset]/YYYYMMDD_HHMMSS_x_dim=8_y_dim=20_alpha=10.0_beta=10.0_ratio=0.2`. - - 2) Model weights will be saved at folder `checkpoint/YYYYMMDD_HHMMSS_x_dim=8_y_dim=20_alpha=10.0_beta=10.0_ratio=0.2`. - - 3) The training loss curves were recorded at folder `graph/YYYYMMDD_HHMMSS_x_dim=8_y_dim=20_alpha=10.0_beta=10.0_ratio=0.2`, which can be visualized using TensorBoard. - - Next, one can run - -```python -python eval.py --data [dataset] --timestamp [timestamp] --epoch [epoch] --train [is_train] -[dataset] - the name of the dataset (e.g.,Splenocyte) -[timestamp] - the timestamp of the experiment you ran -[epoch] - specify to use the results of which epoch (it can be ignored) -[is_train] - indicate training from scratch -``` - -E.g., `python eval.py --data All_blood --timestamp 20200910_143208 --train True` - -The t-SNE visualization plot of latent features (`scDEC_embedding.png`), latent feature matrix (`scDEC_embedding.csv`), inferred cluster label (`scDEC_cluster.txt`) will be saved in the same `results` folder as 1). - - -### Analyzing scATAC dataset without label - -One can also use scDEC to analyze custome scATAC-seq dataset, especially the label is unknown. First, the users should prepare raw read count matrix (`sc_mat.txt`) under the folder `datasets/[NAME]`. `[NAME]` denotes the dataset name. - -Second, one can run the following command: - -```python -python main_clustering.py --data [dataset] --K [nb_of_clusters] --dx [x_dim] --dy [y_dim] --train [is_train] --no_label -[dataset] - the name of the dataset (e.g.,Mydataset) -[nb_of_clusters] - the number of clusters (e.g., 6) -[x_dim] - the dimension of latent space (continous part) -[y_dim] - the dimension of PCA (defalt: 20) -[is_train] - indicate training from scratch -``` - -For an example, one can run `CUDA_VISIBLE_DEVICES=0 python main_clustering.py --data Mydataset --K 10 --dx 5 --dy 20 --train True --no_label` to clustering custom dataset. - -Then one can run `python eval.py --data Mydataset --timestamp YYYYMMDD_HHMMSS --epoch epoch --no_label`. Nota time the timestamp `YYYYMMDD_HHMMSS` (for training) and epoch/batch index `epoch` (the last training epoch/batch index is recommended) should be provided. The clustering results (cluster assignments) will be saved in the `results/Mydataset/YYYYMMDD_HHMMSS_xxx` folder. - - - -## Tutorial - -[Tutorial Splenocyte](https://github.com/kimmo1019/scDEC/wiki/Splenocyte) Run scDEC on Splenocyte dataset (3166 cells) - -[Tutorial Full mouse atlas](https://github.com/kimmo1019/scDEC/wiki/Full-Mouse-atlas) Run scDEC on full Mouse atlas dataset (81173 cells) - -[Tutorial PBMC10k paired data ](https://github.com/kimmo1019/scDEC/wiki/PBMC10k) Run scDEC on PBMC data, which contains around 10k cells with both scRNA-seq and scATAC-seq (labels were manually annotated from 10x Genomic R&D group) - -## Contact - -Also Feel free to open an issue in Github or contact `liuqiao@stanford.edu` if you have any problem in running scDEC. - -## License - -This project is licensed under the MIT License - see the LICENSE.md file for details +# scDEC + +[](https://zenodo.org/badge/latestdoi/286327774) + +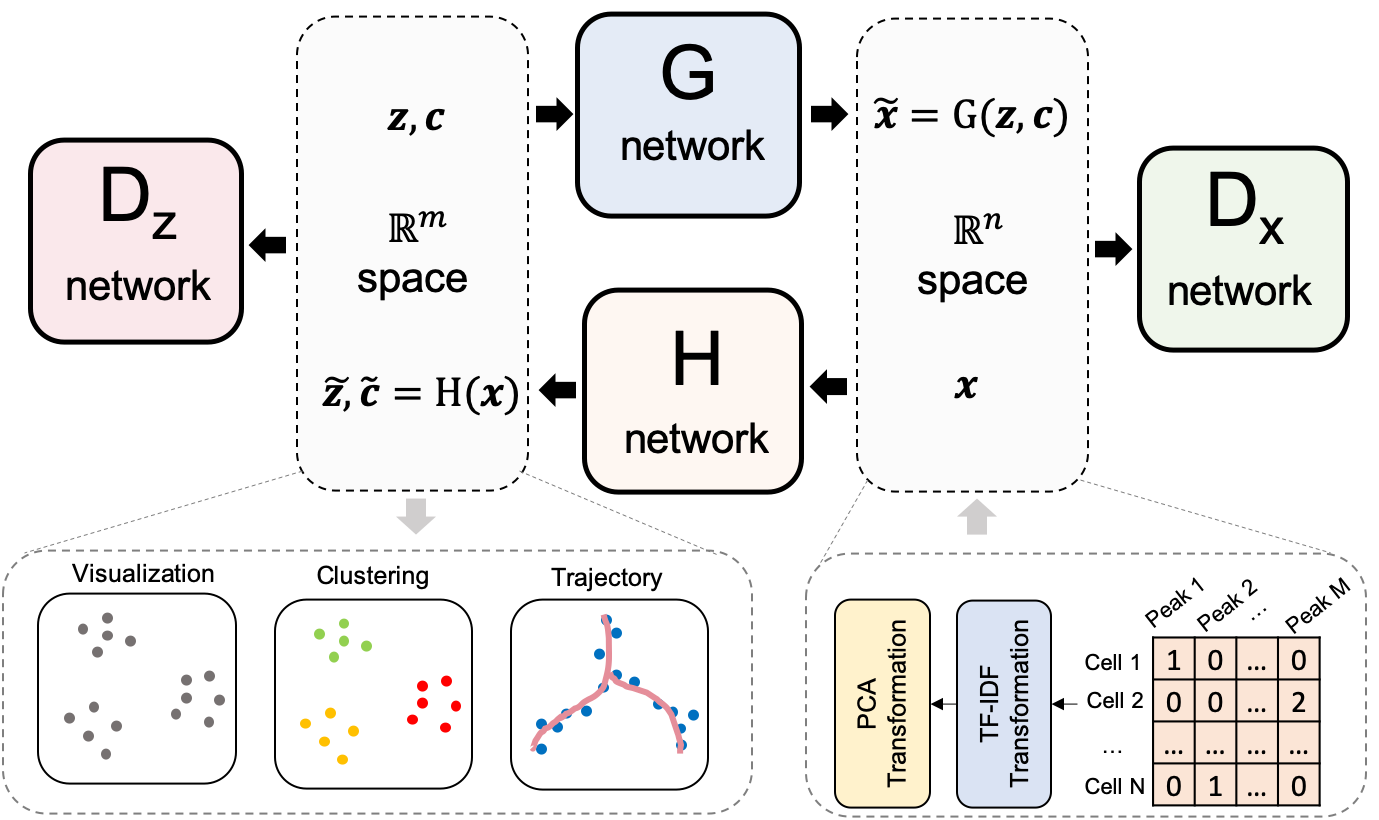 + +scDEC is a computational tool for single cell ATAC-seq data analysis with deep generative neural networks. scDEC enables simultaneously learning the deep embedding and clustering of the cells in an unsupervised manner. scDEC is also applicable to multi-modal single cell data. We tested it on the PBMC paired data (scRNA-seq and scATAC-seq) from 10x Genomics (see Tutorials). + +## Recent News + +An modified version of scDEC won the first place in [NeurIPS 2021 Multimodal Single-Cell Data Integration competition](https://openproblems.bio/neurips_2021/) two Joint Embedding tasks. + +## Requirements +- TensorFlow==1.13.1 +- Scikit-learn==0.19.0 +- Python==2.7 + +## Installation +Download scDEC by +```shell +git clone https://github.com/kimmo1019/scDEC +``` +Installation has been tested in a Linux platform with Python2.7. GPU is recommended for accelerating the training process. + +## Instructions + +This section provides instructions on how to run scDEC with scATAC-seq datasets. One can also refer to [Codeocean platform](https://codeocean.com/capsule/0746056) and click `Reproducible Run` on the right. The embedding and clustering results of several datasets will be shown on the right panel. + +### Data preparation + +Several scATAC-seq datasets have been prepared as the input of scDEC model. These datasets can be downloaded from the [zenode repository](https://zenodo.org/record/3984189#.XzDpJRNKhTY). Uncompress the `datasets.tar.gz` in `datasets` folder then each dataset will have its own subfolder. Each dataset will contain two major files, which denote raw read count matrix (`sc_mat.txt`) and cell label (`label.txt`), respectively. The first column of `sc_mat.txt` represents the peaks information. + +### Model training + +scDEC is an unsupervised learning model for analyzing scATAC-seq data. One can run + +```python +python main_clustering.py --data [dataset] --K [nb_of_clusters] --dx [x_dim] --dy [y_dim] --train [is_train] +[dataset] - the name of the dataset (e.g.,Splenocyte) +[nb_of_clusters] - the number of clusters (e.g., 6) +[x_dim] - the dimension of Gaussian distribution +[y_dim] - the dimension of PCA (defalt: 20) +[is_train] - indicate training from scratch or using pretrained model + +``` +For an example, one can run `CUDA_VISIBLE_DEVICES=0 python main_clustering.py --data Splenocyte --K 12 --dx 8 --dy 20` to cluster the scATAC-seq data with pretrained model. Note that the dimension of the embedding should be `K+x_dim` + +Or one can run `CUDA_VISIBLE_DEVICES=0 python main_clustering.py --data Splenocyte --K 12 --dx 8 --dy 20 --train True` to train the model from scratch. + +### Model evaluation + +If the pretrained model was used, the clustering results in the last step will be directly saved in `results/[dataset]/data_pre.npz` where `dataset` is the name of the scATAC-seq dataset. Note that `data_pre.npz` or `data_at_xxx.npz` contains the predictions from the H network. The first part denotes the embeddings and the second part denotes the inferred one-hot label where one can use `np.argmax` function to get the cluster label. + +Then one can run `python eval.py --data [dataset]` to analyze the clustering results. +For an example, one can run `python eval.py --data Splenocyte` + +The t-SNE visualization plot of latent features (`scDEC_embedding.png`), latent feature matrix (`scDEC_embedding.csv`), inferred cluster label (`scDEC_cluster.txt`) will be saved in the `results/[dataset]` folder. + + +If scDEC model was trained from scratch, the results will be marked by a unique timestamp YYYYMMDD_HHMMSS. This timestamp records the exact time when you run the script. The outputs from the training includes: + + 1) `log` files and predicted assignmemnts `data_at_xxx.npz` (xxx denotes different epoch) can be found at folder `results/[dataset]/YYYYMMDD_HHMMSS_x_dim=8_y_dim=20_alpha=10.0_beta=10.0_ratio=0.2`. + + 2) Model weights will be saved at folder `checkpoint/YYYYMMDD_HHMMSS_x_dim=8_y_dim=20_alpha=10.0_beta=10.0_ratio=0.2`. + + 3) The training loss curves were recorded at folder `graph/YYYYMMDD_HHMMSS_x_dim=8_y_dim=20_alpha=10.0_beta=10.0_ratio=0.2`, which can be visualized using TensorBoard. + + Next, one can run + +```python +python eval.py --data [dataset] --timestamp [timestamp] --epoch [epoch] --train [is_train] +[dataset] - the name of the dataset (e.g.,Splenocyte) +[timestamp] - the timestamp of the experiment you ran +[epoch] - specify to use the results of which epoch (it can be ignored) +[is_train] - indicate training from scratch +``` + +E.g., `python eval.py --data All_blood --timestamp 20200910_143208 --train True` + +The t-SNE visualization plot of latent features (`scDEC_embedding.png`), latent feature matrix (`scDEC_embedding.csv`), inferred cluster label (`scDEC_cluster.txt`) will be saved in the same `results` folder as 1). + + +### Analyzing scATAC dataset without label + +One can also use scDEC to analyze custome scATAC-seq dataset, especially the label is unknown. First, the users should prepare raw read count matrix (`sc_mat.txt`) under the folder `datasets/[NAME]`. `[NAME]` denotes the dataset name. + +Second, one can run the following command: + +```python +python main_clustering.py --data [dataset] --K [nb_of_clusters] --dx [x_dim] --dy [y_dim] --train [is_train] --no_label +[dataset] - the name of the dataset (e.g.,Mydataset) +[nb_of_clusters] - the number of clusters (e.g., 6) +[x_dim] - the dimension of latent space (continous part) +[y_dim] - the dimension of PCA (defalt: 20) +[is_train] - indicate training from scratch +``` + +For an example, one can run `CUDA_VISIBLE_DEVICES=0 python main_clustering.py --data Mydataset --K 10 --dx 5 --dy 20 --train True --no_label` to clustering custom dataset. + +Then one can run `python eval.py --data Mydataset --timestamp YYYYMMDD_HHMMSS --epoch epoch --no_label`. Nota time the timestamp `YYYYMMDD_HHMMSS` (for training) and epoch/batch index `epoch` (the last training epoch/batch index is recommended) should be provided. The clustering results (cluster assignments) will be saved in the `results/Mydataset/YYYYMMDD_HHMMSS_xxx` folder. + + + +## Tutorial + +[Tutorial Splenocyte](https://github.com/kimmo1019/scDEC/wiki/Splenocyte) Run scDEC on Splenocyte dataset (3166 cells) + +[Tutorial Full mouse atlas](https://github.com/kimmo1019/scDEC/wiki/Full-Mouse-atlas) Run scDEC on full Mouse atlas dataset (81173 cells) + +[Tutorial PBMC10k paired data ](https://github.com/kimmo1019/scDEC/wiki/PBMC10k) Run scDEC on PBMC data, which contains around 10k cells with both scRNA-seq and scATAC-seq (labels were manually annotated from 10x Genomic R&D group) + +## Contact + +Also Feel free to open an issue in Github or contact `liuqiao@stanford.edu` if you have any problem in running scDEC. + +## License + +This project is licensed under the MIT License - see the LICENSE.md file for details

 Datasets
Datasets
 Models
Models